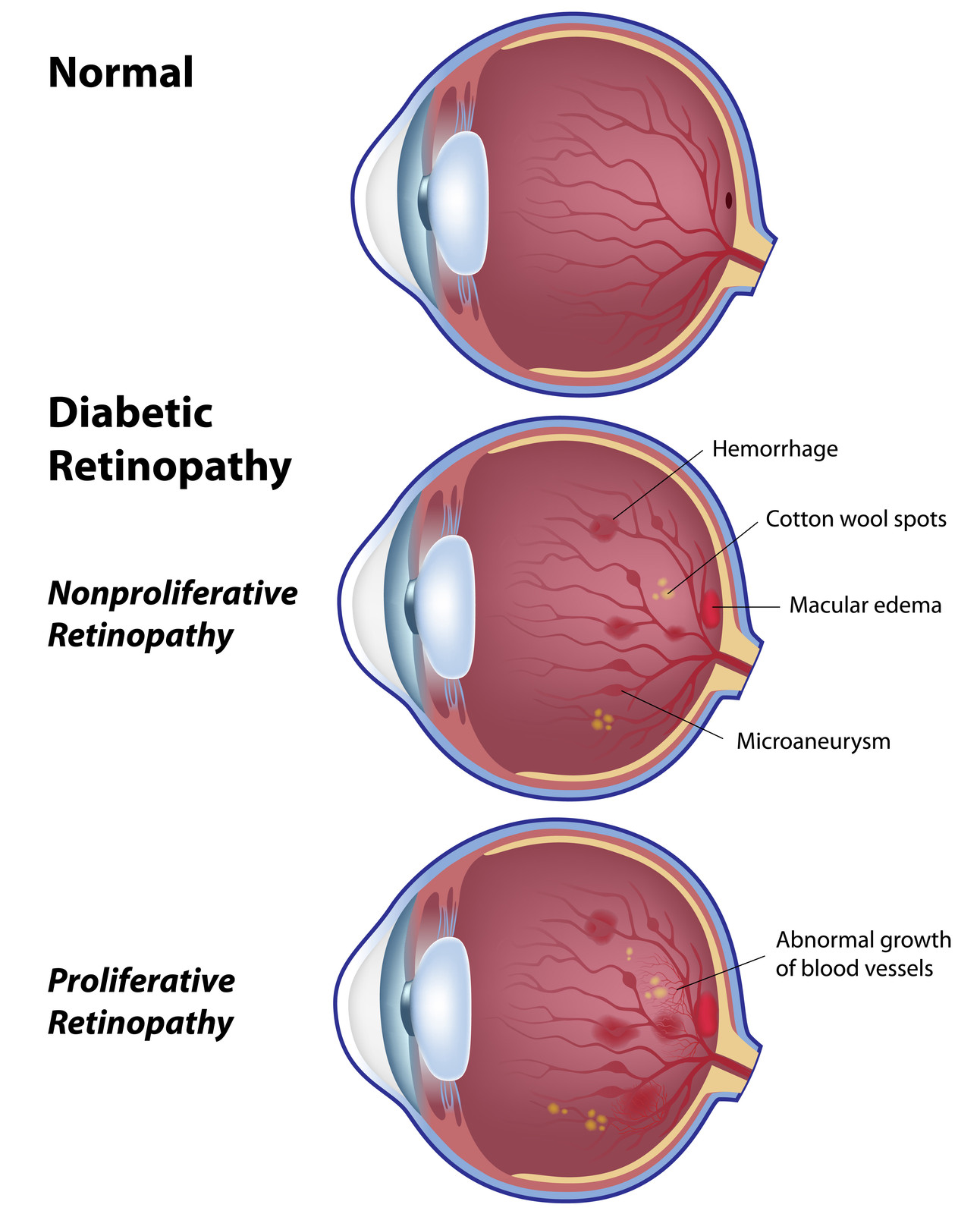
10 Jan Breakthrough in Treatment of Diabetic Retinopathy
Breakthrough Results of the DRCR.net Protocol S and Protocol T Will Dramatically Change the Treatment of Diabetic Retinopathy
The Diabetic Retinopathy Clinical Research Network (DRCR.net) is a collaborative network dedicated to facilitating multicenter clinical research of diabetic retinopathy, diabetic macular edema and associated conditions; some of the leading causes of vision loss. The DRCR.net supports the identification, design, and implementation of multicenter clinical research initiatives focused on diabetes-induced retinal disorders. The idea was to develop a network of clinical practices to harness the ability of these practices to recruit large number of patients rapidly and to study these patients by centralizing data through the internet.
Principal emphasis is placed on clinical trials, but epidemiologic outcomes and other research may be supported as well. The DRCR.net was formed in September 2002 and currently includes over 115 participating sites (offices) with over 400 physicians throughout the United States. The DRCR.net is funded by the National Eye Institute (NEI). The NEI is a part of the National Institutes of Health, which is the branch of government that funds medical research.
Protocol T compared the efficacy of ranibizumab, bevacizumab and aflibercept in the treatment of diabetic macular edema. The results were published in the New England Journal of Medicine this year (Wells J, Glassman A, Ayala A, The Diabetic Retinopathy Clinical Research Network. Aflibercept, bevacizumab or ranibizumab for diabetic macular edema. N Engl J Med 2015;26;372:13:1193-203). For patients with vision better than 20/40 there was no difference between the three drugs at one year but for vision worse than 20/50 aflibercept patients had more vision improvement (18.9 letters) compared to bevacizumab (11.8) or ranibizumab (14.2). These results suggest that the least expensive drug should be used for patients with 20/40 or better vision but that the most expensive drug should be used for patients with 20/50 or worse vision.
Protocol S compared the efficacy of at least 4 monthly injections of ranibizumab to laser pan retinal photocoagulation for the treatment of proliferative diabetic retinopathy. The results were presented just recently at the 2015 Annual meeting of the American Academy of Ophthalmology and published in JAMA Ophthalmology minutes before the presentation (Gross JG. DRCR.net Prompt PRP vs ranibizumab+deferred PRP for PDR study (Protocol S).
Presented at: American Academy of Ophthalmology. Nov. 13, 2015; Las Vegas) All patients received 6 monthly injections the first year and about half of that in the second year. At 24 months, ranibizumab treatment was not inferior to laser. Moreover, the vision was better in the ranibizumab treated eyes, there was less visual field defect and less diabetic macular edema. This may signal the end to laser pan retinal photocoagulation for those patients with proliferative diabetic retinopathy, who can afford intravitreal injections and who are willing to be followed closely.
By utilizing the ability of small clinical sites to recruit large numbers of patients, and centralized data processing to analyze the massive amount of clinical data generated, the DRCR.net supports efficient, effective and, most importantly, population wide clinical data on the treatment and management of diabetes related eye diseases.
Protocol T was able to identify a sub-population of patients with DME that can be well managed using lower-cost but equally effective drug therapies; a huge benefit when you consider that ranibizumab accounted for nearly 10% of the entire medicare part B drug budget and was the program’s single largest expenditure (2010). Furthermore, protocol S identified that PDR patients can be better managed with drug therapy rather than traditional laser therapy which have more side-effects, longer recovery time, and poorer visual outcomes.
These two examples illustrate how a collaborative effort from multiple small clinical sites can provide new insights into complicated diseases such as diabetes related eye-diseases. And that these efforts can lead to the necessary reduction of healthcare related costs without reducing efficacy, can identify relevant therapies for relevant populations, and can compare new and emerging therapies with outcomes from existing therapies.
Lawrence P. Chong, MD
Cinical Professor of Ophthalmology
Keck School of Medicine
Retina Specialist, VMR Institute, Huntington Beach CA

